MICROPTIC SL, based in Barcelona, Spain, is a leading player in the field of research, development of automatic diagnostic systems, having specialized in SEMEN analysis for over 20 years. It is an ISO 9001 & ISO 13485 certified company using artificial vision technology & specialized in biomedicine.


SCA® is a modular automatic system for the analysis of semen samples, according to pre-defined criteria,
for most of the vertebrates (mammals, avian, reptile, amphibian and fish) and invertebrates (e.g. echinoderms and molluscs). Complete modular system allows you to choose modules as per your requirement and compatible accessories.
SCA Veterinary CASA System for semen analysis allows the accurate, repetitive and automatic assessment of the following sperm parameters: Motility & Concentration, Morphology, DNA fragmentation, Vitality, Acrosome reaction.
| Motility and concentration are analyzed in Phase contrast microscopy (an optical microscopy technique that converts phase shifts in light passing through a transparent specimen to brightness changes in the image These phase shifts, although invisible, become visible when shown as brightness variations allowing the clear visualization of the semen sample). |
| Or Fluorescence (The specimen is illuminated with light of a specific wavelength which is absorbed by the fluorochromes, causing them to emit light of longer wavelengths (i.e., of a different color than the absorbed light) |
| Images are captured with high frame rate digital camera Basler (25 , 50, 75 or up to 200 fps) |
| Automatic detection of non homogeneous fields, allowing a consistent analysis |
| There is a sort function that permits to create groups of sperm population, useful in any sperm research. |
| Modifiable parameters: Particle area for sperm detection, Velocity Classification, VAP, Frame rate, Connectivity, Circularity, Parameter restriction, for example as elongation (to improve the cell detection) |
| Other modifiable parameters: color mask of the path. |
| Allows capture up to 30 fields of the sample |
| Capture time is 1 second per field |
| Intelligent filter that automatically eliminate captured debris in human semen samples |
| Compatible with several disposable analysis chambers like Leja® or Makler® counting chamber |
Kinematic parameters:
VCL : curvilinear velocity (m/s). Time-averaged velocity of a sperm head along its actual curvilinear path, as perceived in two dimensions in the microscope. A measure of cell vigour.
VSL : straight-line (rectilinear) velocity (µm/s). Time-averaged velocity of a sperm head along the straight line between its first detected position and its last.
VAP : average path velocity (µm/s). Time-averaged velocity of a sperm head along its average path. This path is computed by smoothing the curvilinear trajectory according to algorithms in the SCA Motility module.
ALH : amplitude of lateral head displacement (µm). Magnitude of lateral displacement of a sperm head about its average path. It can be expressed as a maximum or an average of such displacements.
LIN: linearity. The linearity of a curvilinear path, VSL/VCL.
WOB: wobble. A measure of oscillation of the actual path about the average path, VAP/VCL.
STR : straightness. Linearity of the average path, VSL/VAP.
BCF: beat-cross frequency (Hz). The average rate at which the curvilinear path crosses the average path.
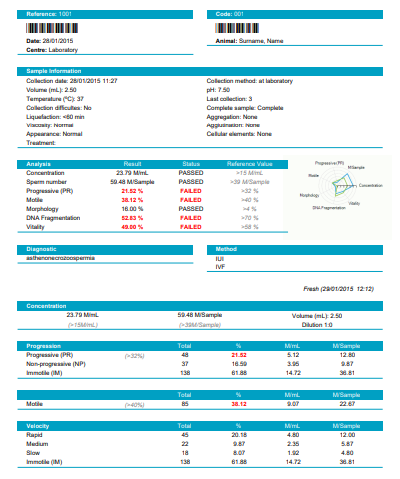
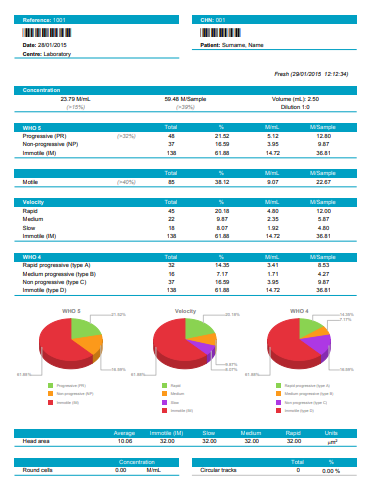
| Sample (ejaculate or straws) Concentration in M/ml. |
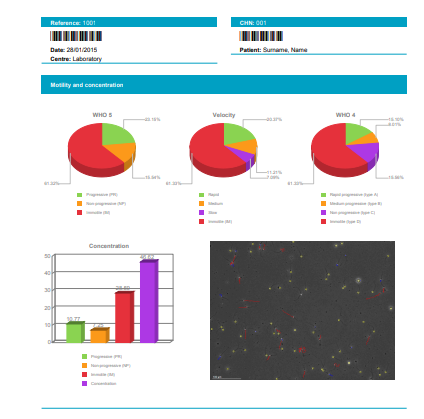
| Total analysed spermatozoa , Percentage in the sample, concentration in M/ml and in the sample volume of the spermatozoa classified by: |
| Velocity: Rapid, Medium, slow and Static |
| Classification: Type A (rapid and progressive), B (medium progressive), C(non progressive) and D (static or immotile) |
| Classification: Progressive (Type A+B), Motile (Type A+B+C), Static (Type D) |
| Hyperactive |
| Average of the kinematic parameters presented in the sample |
| Total of the head Area / velocity type |
| Total of Round cells / velocity type |
| Total and percentage of circular tracks |
| ALH and BCF in Medium and Rapid Progressive Sperm |
| Analysis under brightfield microscopy, transmitting light to view a specimen that is stained. |
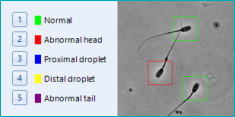
| SCA Morphology analyzes the head, acrosome and midpiece form, shape and size. |
| Tails can be analyzed automatically with the use of the specific high resolution digital camera 1280×1024 or manually if desired. |
| Automatic vacuoles analysis is available using the Sperm Blue® staining kit. |
| There are several Compatible staining methods depending on the specimen: Diff quik, Papanicoulau, Sperm Blue®, Sperm Stain, Shorr and CellVu prestained slides for human. Sperm Blue® for all species, which allows a complete analysis, including acrosome visualization. For each of the staining used the software has corrected factors. |
| Allows the creation of User defined matrix for the classification into normal or abnormal sperm |
| Additional descriptions of the Morphologic values can be created |
| Modifiable parameters are: Size of the capture box, staining methods and Classification criteria |
| SCA® permits to capture and analyse up to 500 spermatozoa per sample |
| Head length, width, area, perimeter, ellipticity, elongation, roughness, regularity |
| Acrosome, percentage of head size |
| Vacuoles, presence and percentage of head size (with SpermBlue® staining) |
| Midpiece width, area, insertion angle |
| Tail length |
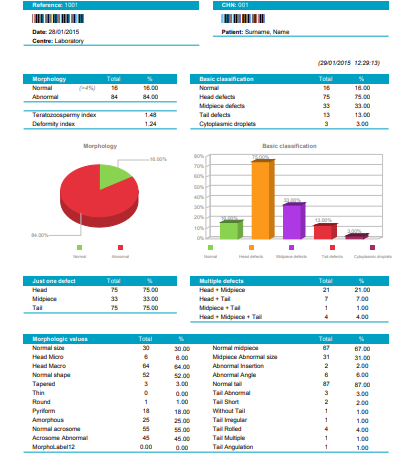
| Calculation of the following basic parameters: Total analyzed and Percentage of normal and abnormal spermatozoa |
| Total analyzed and Percentage of normal spermatozoa; with head, midpiece and tail defects; and cytoplasmic droplets. |
| Total and percentage of counted spermatozoa having Just one defect, in the Head, Midpiece or Tail. |
| Total and percentage of counted spermatozoa having Multiple defects combined as: Head+ Midpiece, Head+Tail, Midpiece+Tail, Head+Midpiece+Tail |
| Morphologic values*: Total and percentage of analysed spermatozoa having Normal size, Micro, Macro, Normal shape, Tapered, Thin, Round, Pyriform, Amorphous; Normal and Abnormal Acrosome; Normal midpiece, Abnormal midpiece size, angle and insertion; Normal tail, abnormal, short, Irregular, Rolled, Multiple, Without, Angulation |
| Average and deviation of these morphometric values: Head lengh, width, area, perimeter, ellipticity, elongation, roughness, regularity; Midpiece width, area and angle; Acrosome |
| Teratozoospermy index (TZI), Deformity index(SDI), Multiple anomalies index (MIA), Number of spermatozoa with Excess residual cytoplasm (ERC) |
| DNA fragmentation can be analysed under brightfield microscopy, transmitting light to view a specimen that is stained. |
| Or Fluorescence (The specimen is illuminated with light of a specific wavelength which is absorbed by the fluorochromes, causing them to emit light of longer wavelengths (i.e., of a different color than the absorbed light) |
| Disposables: Halosperm® kit for human or any preparation based in the Sperm Chromatin Dispersion (SCD). |
| SCA permits to capture and analyse up to 500 spermatozoa per sample |
| Counting of fragmented and non fragmented spermatozoa by halo area or halo-core ratio automatic assessment. |
| Basic results: Number and percentage of fragmented and non fragmented spermatozoa |
| Advanced parameters: Number and percentage of spermatozoa having big, medium, small hallo, degraded and without hallo are analyzed |
| The assessment is under fluorescence microscopy at 20x: The specimen is illuminated with light of a specific wavelength, which is absorbed by the fluorochromes, causing them to emit light of longer wavelengths, i.e., of a different color than the absorbed light . |
| Vitality of spermatozoa is evaluated indirectly though the integrity of the cellular membrane using vital fluorescence staining FluoVit®. |
| Vitality analysis can be also assessed in brightfield at 20x using the Vitality slides SCA. |
| Possibility to add or eliminate spermatozoa and/or captures fields |
| Calculation of basic parametres: Number and percentage of live and dead spermatozoa. |
| Visualization of the analysis mask superimposed to the original image. |
| Image capture takes 1 second per field |
| Allows capture up to 30 fields of the sample |
| Counting of live and dead detection by color detection. |
| Number and percentage live and dead spermatozoa. |
| The assessment is under fluorescence microscopy at 40x (Plan Fluor): The specimen is illuminated with light of a specific wavelength, which is absorbed by the fluorochromes, causing them to emit light of longer wavelengths, i.e., of a different color than the absorbed light . |
| Acrosome reaction of spermatozoa is evaluated using FluoAcro kit. |
| Possibility to add or eliminate spermatozoa and/or captures fields |
| Calculation of basic parametres: Number and percentage of spermatozoa with acrosome intact and acrosome reacted. |
| Visualization of the analysis mask superimposed to the original image. |
| Image capture takes 1 second per field |
| Allows capture up to 30 fields of the sample |
| Counting of spermatozoa with acrosome intact and acrosome reacted by color detection. |
| Number and percentage of spermatozoa with acrosome intact and acrosome reacted. |
Motility & concentration as well as droplets and coiled tails are rapidly and automatically analyzed with SCA Production. Also acrosome integrity can be automatically analyzed
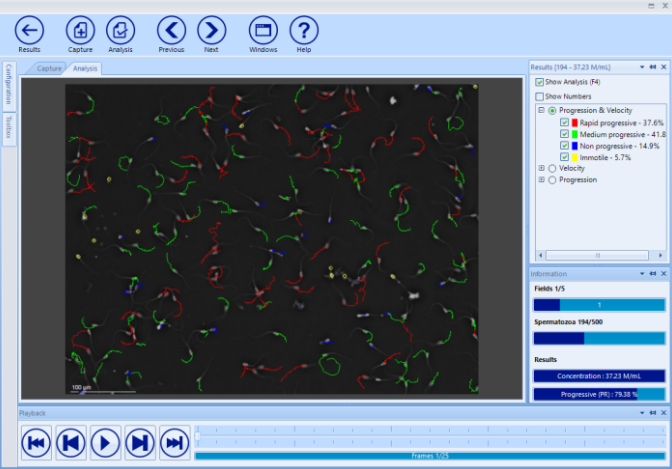

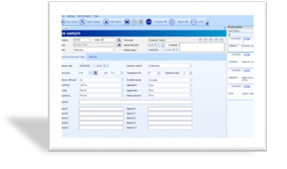
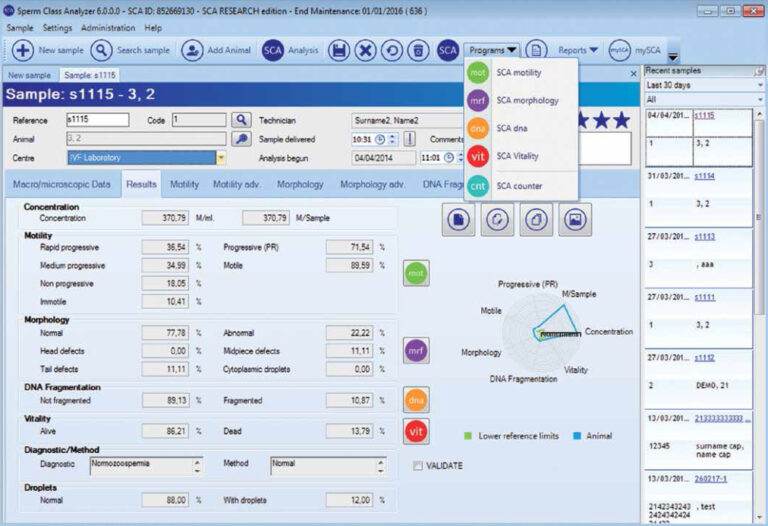
Working with the SCA® database (SQL server), review results and print reports (more than 40 predefined and possible to customize).
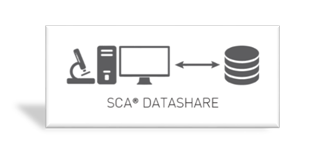
Used to connect SCA® database to any external database or laboratory information system.

For a fully automatic system. Uses a motorized stage.
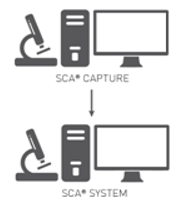
Low price option for centres with main lab and secondary labs, or a requirement for more than one CASA system.
Software to modify SCA® sessions. The sessions must be analyzed by FULL SCA® system. Not possible to capture/import new fields.
Open and view all the saved sessions from any computer. Send the file to you patients and they can see the results in their home. This software is free!

Stay in touch with us to get latest news and special offers.
All brand names are copyright © of the companies that have been listed. All Rights Reserved.